
PFAS Analytical Methods
Dr. Sara Nason
Connecticut Agricultural Experiment Station
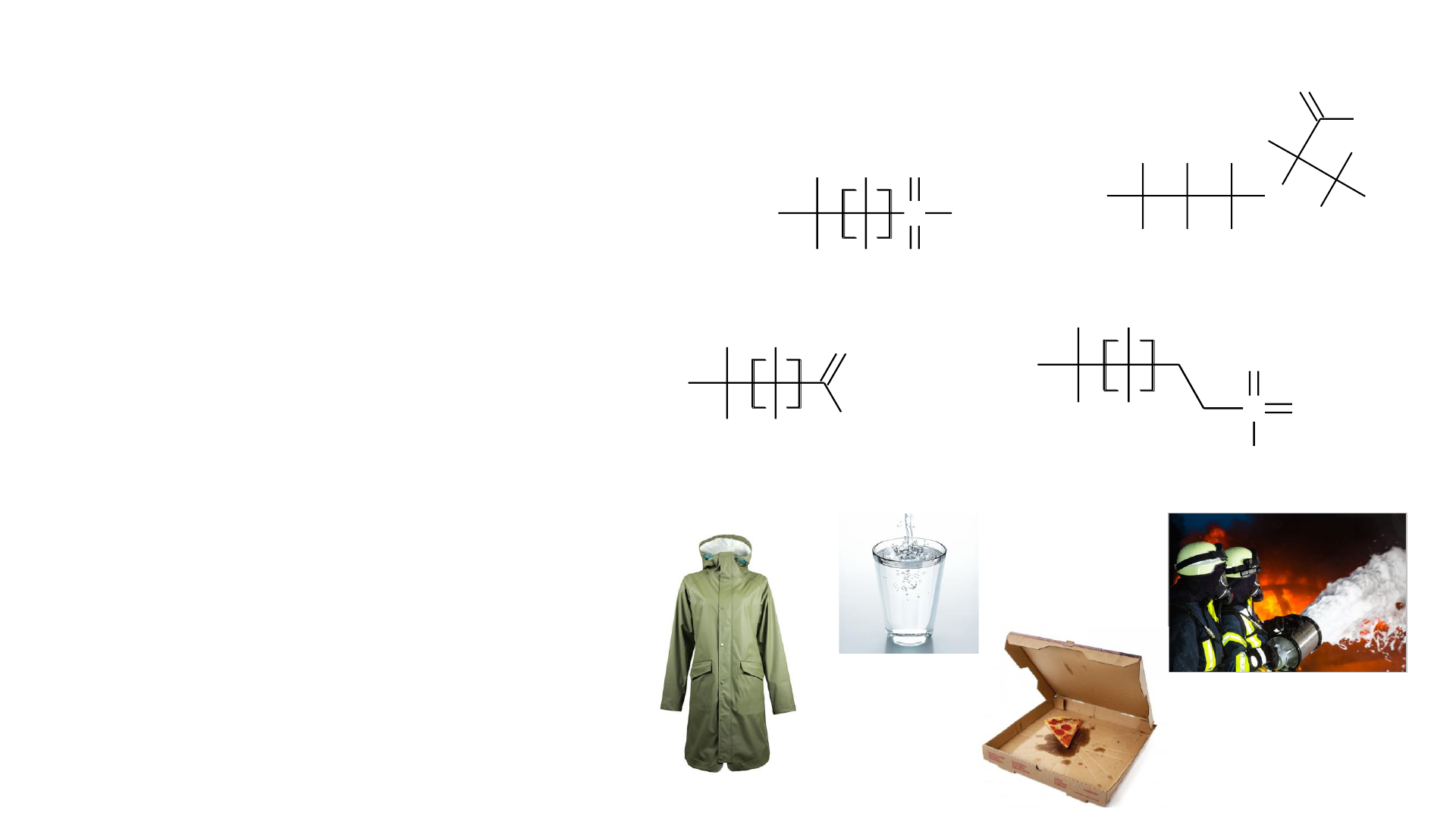
PFAS: Per- and Polyfluoroalkyl Substances
• Molecules that contain carbon-
fluorine chains
• Properties depend on chain length and
headgroup
• Over 8,000 listed on US EPA website
• “Forever Chemicals”
• In use since the 1940s
• Used in many common consumer
products
• Widely spread environmental
contaminants
SF
F
F
F
F O
O
OH
n
F
F
F
F
F
O
OH
n
O
F
F
F
F
F
F
F
F
F
F
F
OH
O
F
F
F
F
F
S
OH
O
O
n

PFAS: Toxic Effects
• Highly toxic at very low concentrations
• Require very sensitive analysis –
regulatory limits in ppt, most sensitive
methods in ppq
• Pregnancy complications and
neurodevelopmental effects
• Thyroid hormone effects
• Related to high cholesterol
• Can affect the immune system and
response to vaccines

PFAS in the News
Fred Stone, Farm Owner (news.bloomberglaw.com)

PFAS in the News
• Up to 250 ppt PFAS found in Anvil 10+10 – pesticide used in
widespread spraying for mosquitos
• PFAS leached into product from fluorinated HDPE containers
• US EPA is conducting further investigation
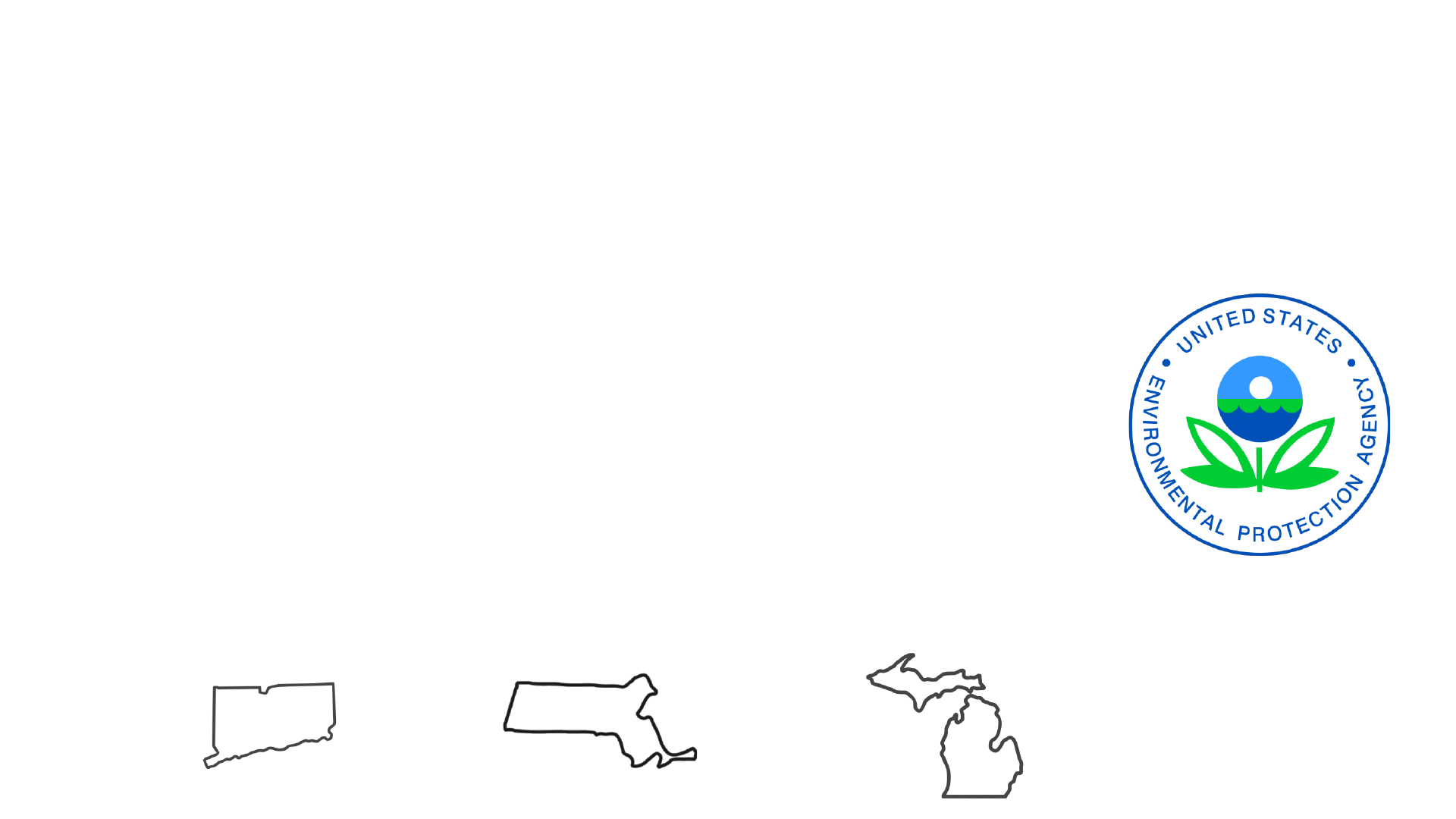
Current PFAS Governance
• Federal: Health Advisory level of 70 ppt for PFOS and PFOA in drinking water
• Recently announced intent to regulate PFOS and PFOA under the Safe Drinking Water
Act based on finding from the fourth Contaminant Candidate List
• 24 States have guidance on PFAS in water
• CT: Drinking Water Action Level 70 ppt (sum of 5 PFAS)
• MA: Drinking Water MCL 20 ppt (sum of 6 PFAS)
• MI: Drinking Water MCLs for 7 individual PFAS (range 6-400,000 ppt)
• Many also have soil guidance, interest in biosolids, biota, food, other media

Existing PFAS Methods (Regulatory)
• EPA methods for Drinking Water
• 537: 14 PFAS (2009)
• 537.1: 18 PFAS (2018)
• SPE and LC-MS/MS
• 533: 25 PFAS (2019)
• SPE and LC-MS/MS, isotope dilution
• US FDA method: 16 PFAS (2019)
• Lettuce, milk, bread, fish
• QuEChERS, dSPE, SPE, LC-MS/MS, isotope dilution
• DoD QSM guidelines
• ELAP certification
• Based on ISO:17025 guidelines

PFAS – general considerations
• PFAS are everywhere
• Materials that can leach PFAS:
• PTFE and other fluorinated plastics (most plastics)
• Stain-proof or waterproof coatings on carpets, shoes,
raingear, lab coats, etc.
• Non-stick coatings
• Paint, car wax
• Fabric softeners/dryer sheets
• Aluminum foil
• PFAS sorb to many materials
• Materials that can remove PFAS from samples:
• Glass
• Most plastics
• Sample filters

PFAS – general considerations
• Generally ok to use:
• Polypropylene (PP) and high-density polyethylene (HDPE)
• Includes most plastic pipette tips and centrifuge tubes
• Storage bottles, autosampler vials, etc. available
• Uncoated stainless steel (spatulas, etc.)
• Glass (select uses only)
• For solvent, buffer, etc. storage (containers that do not touch samples)
• For storage of high concentration standards in organic solvent
• Cotton clothing and lab coats
• Sharpie markers
• LC-MS grade solvents
• Always use full method blanks to check for contamination

PFAS Sample Collection
• Don’t contaminate the samples
• Don’t remove the PFAS
• Use PFAS free containers (not glass)
• Wear cotton clothes
• Take field blank samples
• Keep samples away from potential contamination sources
• Sample storage: -20 °C
• Sorption larger potential problem than degradation

PFAS Sample Preparation - Water
• Direct injection
• Works well with very sensitive instruments
• Smaller chance for sample contamination
• Higher detection limits than SPE
• Solid Phase Extraction
• Be aware of PTFE parts of SPE manifolds (should be refitted)
• EPA 537 and 537.1: SDVB cartridges
• Best for anionic, hydrophobic PFAS
• EPA 533: weak anion exchange cartridges
• Best for anionic PFAS, broader coverage of short chain, hydrophilic PFAS than SDVB
• Evaporation in heated water bath under nitrogen and reconstitution
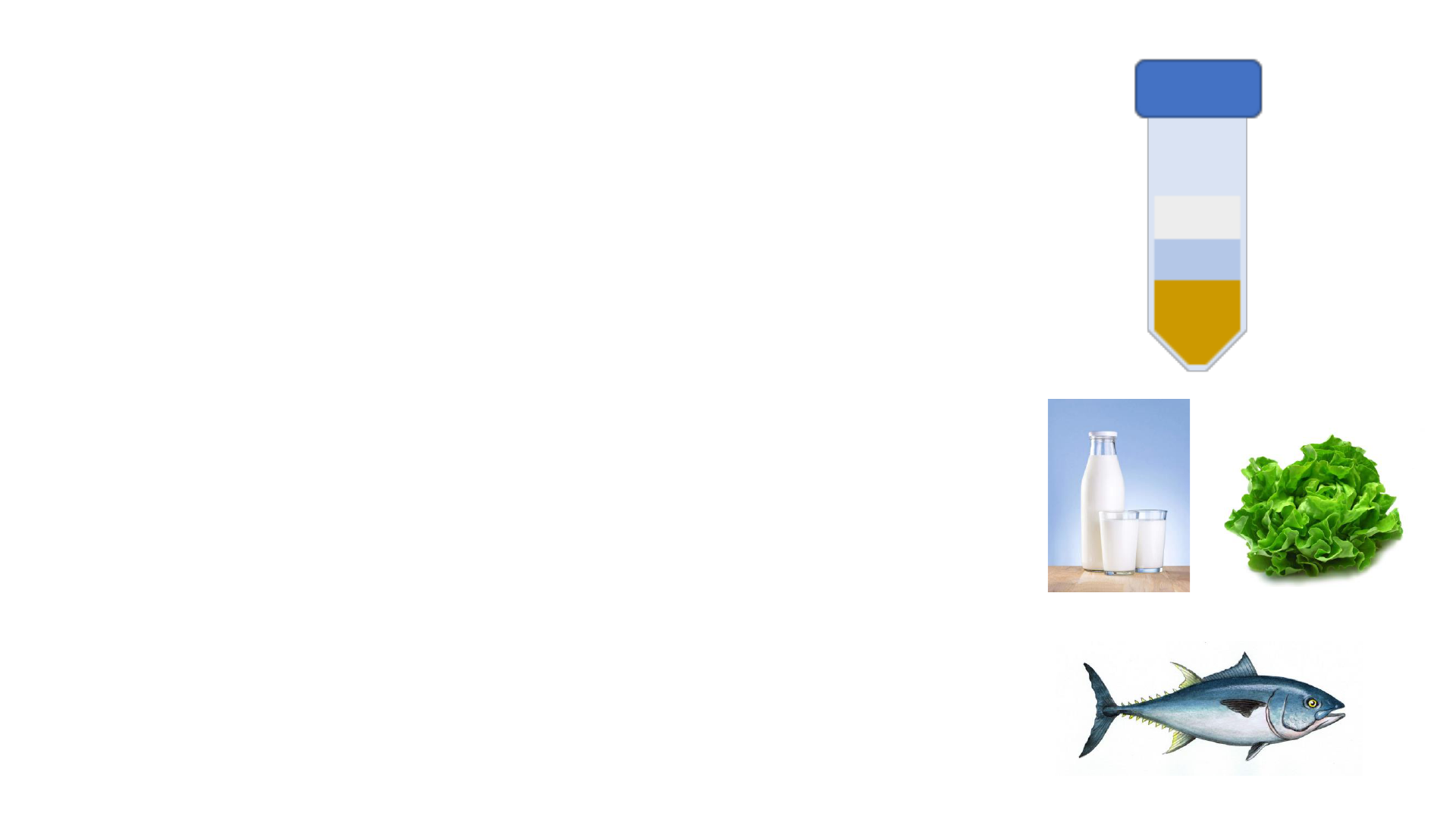
PFAS Sample Preparation - Food
• FDA method: Modified QuEChERS
• Extraction with water, acetonitrile, MgSO
4
, NaCl
• dSPE clean-up with PSA and GCB
• If PFAS detected: second clean-up with weak anion
exchange SPE and rerun to confirm results
• Solvent volumes used and N
2
dry-down are matrix
dependent
• Difficult with fatty matrices (cheese, dog food)

PFAS Sample Preparation – Soil+
• Extraction with buffered methanol
• NaOH very common – good for anionic PFAS
• CH
3
COONH
4
– wider range of PFAS but difficult dry down
• Clean up with GCB
• Evaporation in heated water bath under nitrogen
• Very adaptable for other matrices:
• Have tried in plant tissue, animal feed, dried blood spots

PFAS Sample Preparation - Filters
• Most filter types have the potential to significantly
sorb/leach PFAS
• Bad: nylon, PTFE, anything fluorinated
• Better: regenerated cellulose, PP, HDPE, glass fiber
• Always test new products to ensure compatibility with
method
• Consider pre-rinsing filters
• Option: inject without filtering
• Centrifuge samples at high speed for a long time, at analysis
temperature
• Still presents some risk to instruments

PFAS Analysis – LC
• General method – C18 column with gradient
• HILIC or other useful for especially small/polar PFAS
• Potential contamination sources
• Mobile phases and frits
• Tubing
• Pump Seals
• PTFE vial caps
• Vendor kits available to minimize PFAS on LC
• Use a delay column to separate mobile phase and
pump contamination from PFAS in samples
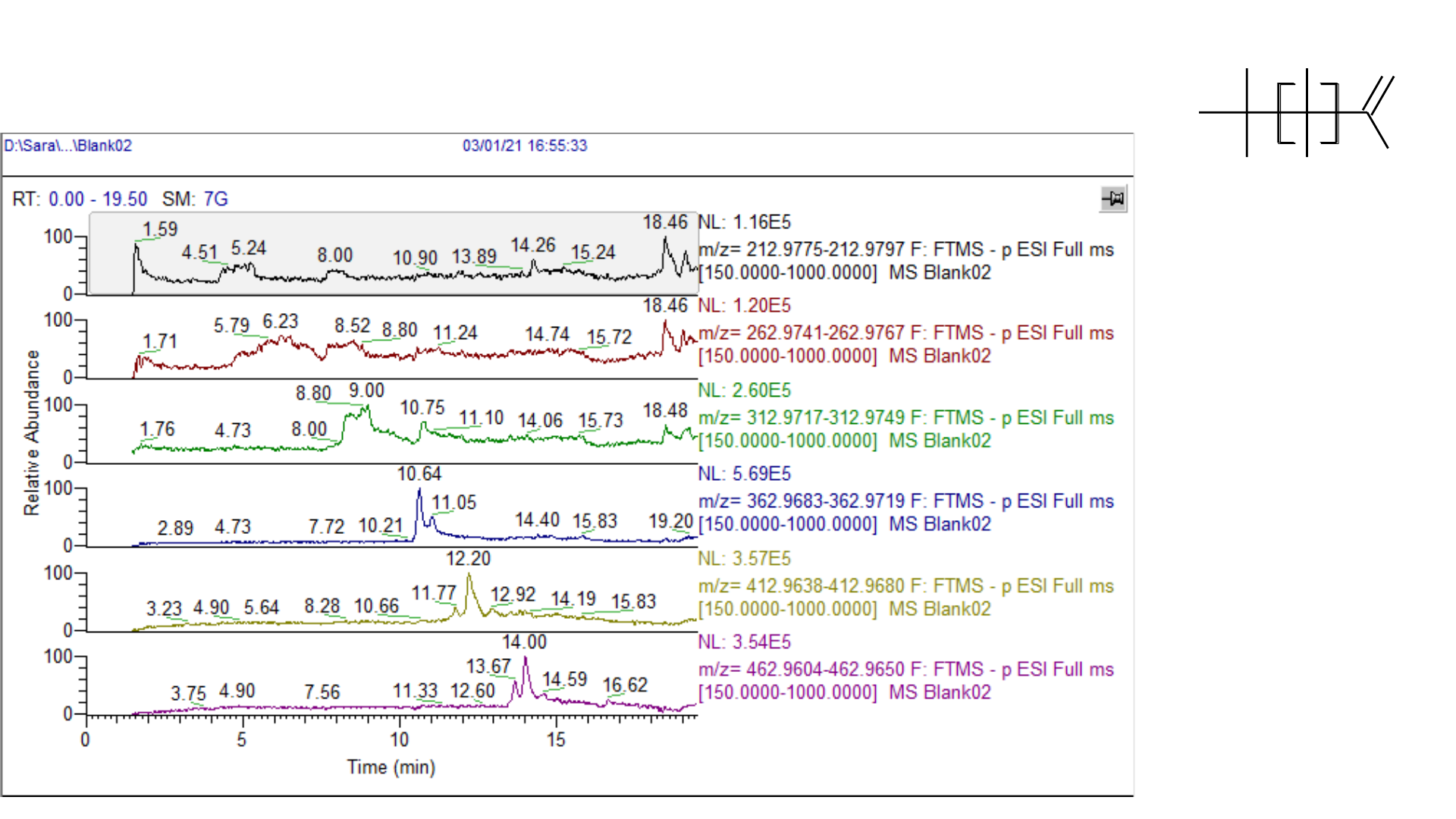
Solvent Blank: PFCA Contamination
F
F
F
F
F
O
OH
n
PFBA
PFPeA
PFHxA
PFHpA
PFOA
PFNA
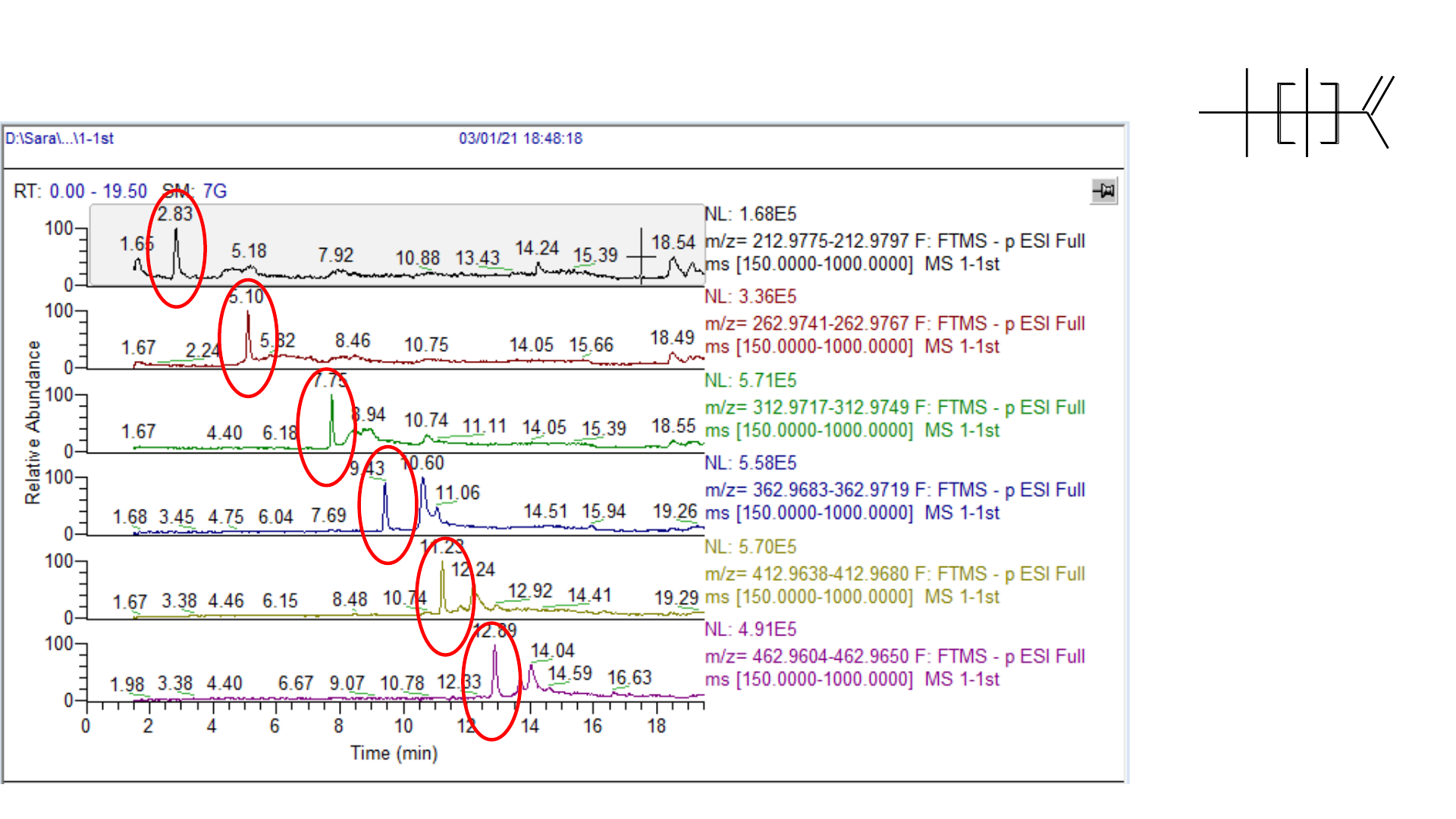
1 ppb standard: PFCAs with Delay Column
F
F
F
F
F
O
OH
n
PFBA
PFPeA
PFHxA
PFHpA
PFOA
PFNA
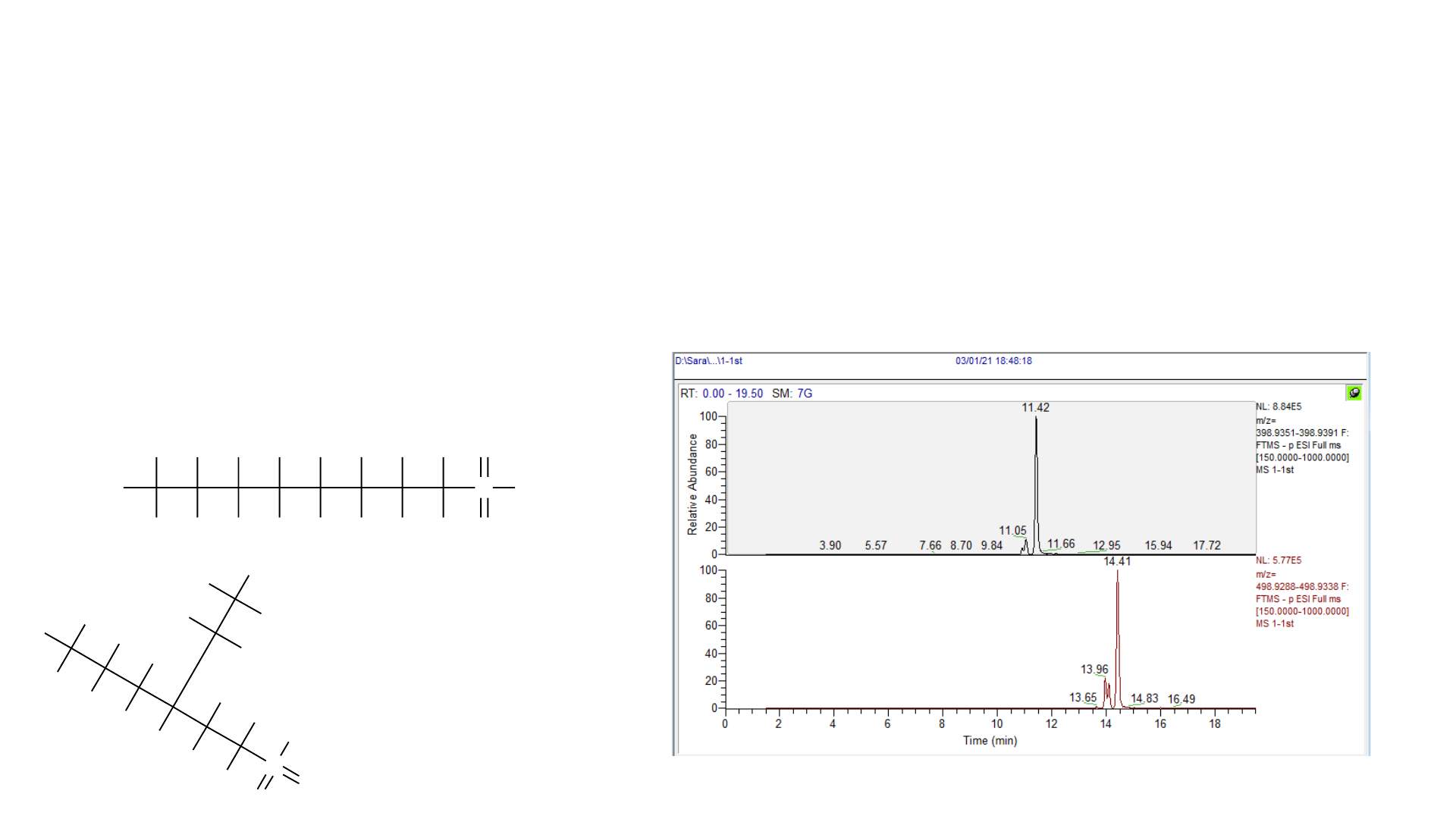
PFAS Analysis – LC
• Challenge: Linear vs. Branched isomers
• Hard to tell apart on MS, but separate chromatographically
• Most methods combine all isomers in one integration for quantitation
• Can buy standards with linear only or a mix
• Mix is often required
• Especially relevant for sulfonic acids
S OH
O
OF
F
F
F
F F
FF
F
F
F
FF
FF
F
F
S
OH
O
O
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
F
Linear and
branched PFOS
PFHxS
PFOS
PFAS Analysis – LC-MS/MS
• Triple quadrupole: lowest detection limits
• Parent and fragment ions necessary, with ratio
• Interference possible!
• Targeted screening only
• ESI- used for most commonly analyzed PFAS
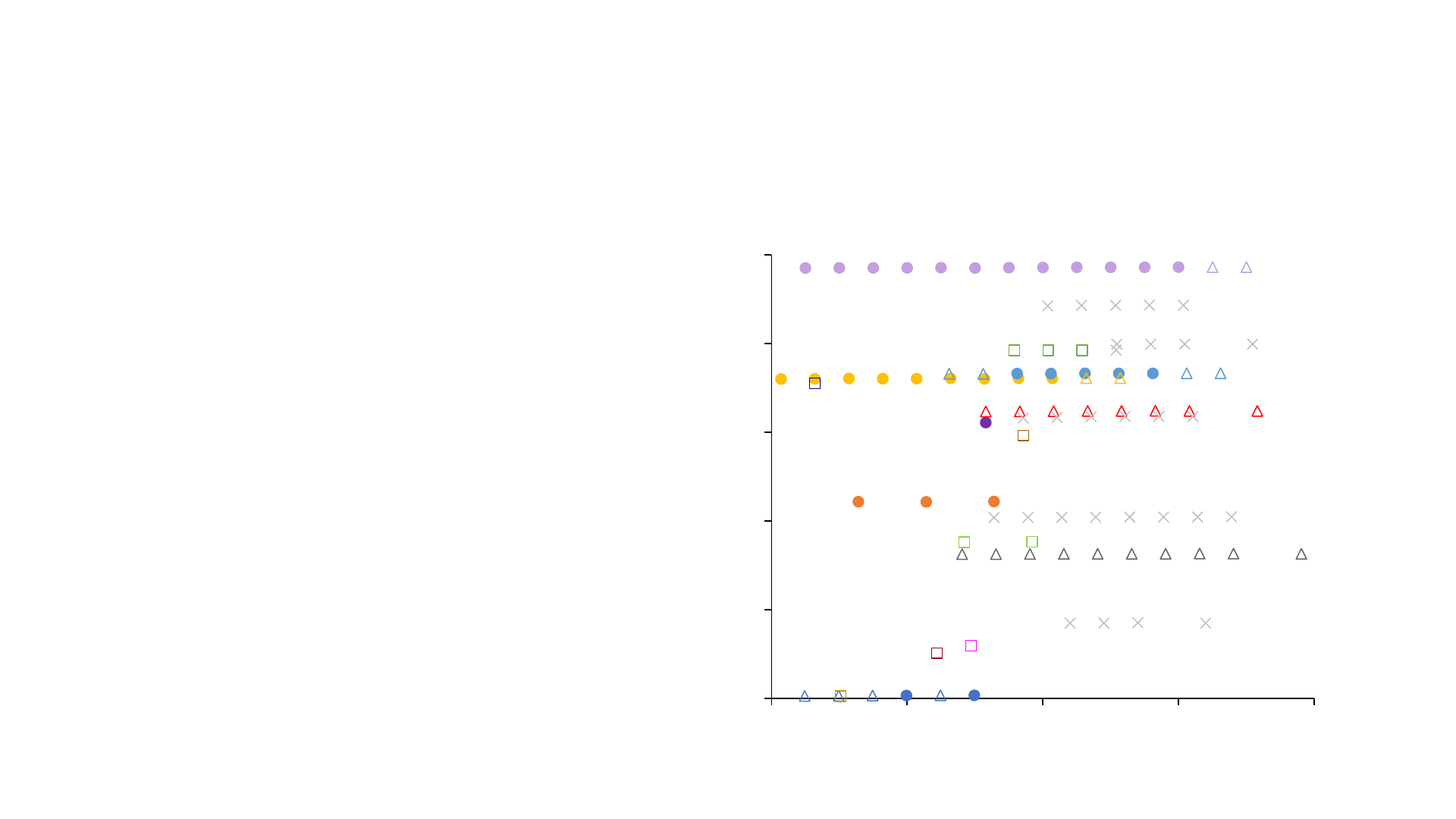
PFAS Analysis – LC-HRMS
• Higher detection limits
• Detection based on exact mass
– fragments less important
• Potential for non-targeted
screening
• FluoroMatch Software
• Kendrick mass defect analysis
0
0.2
0.4
0.6
0.8
1
200 400 600 800 1000
CF2 Kendrick Mass Defect
Molecular Weight
SA
FT SN X:1
Sulfonamide-N-methyl alkyl acid
FT sulfone
FT SN X:2-OH
H-substituted unsaturated SA
FT thioether
Pentafluorosulfide SA
Carboxylic acid
Unsaturated SA
SA ether
Chlorinated SA
FT SN X:2
Bis(perfluorosulfonyl)amine
Sulfonamide

PFAS – Other Analysis
• TOP (Total Oxidizable Precursor) assay
• Total Organic Fluorine
• PFAS analysis by GC-MS/MS
F
F
F
F
F
S
OH
O
O
n
Fluorotelomer sulfonate X:2
F
F
F
F
F
O
OH
n
Carboxylic Acid

PFAS standards
• Native standards widely available for most common PFAS
• Wellington labs has the broadest coverage
• Mass-labeled standards
• Wellington Labs has best selection
• Cambridge isotopes has some
• Many PFAS have no standards available

Conclusions
• Evaluate all PFAS-related supplies very carefully – PFAS are everywhere!
• Use blanks appropriately
• Isotope dilution is best
• Outfit you LC appropriately
• LC-QQQ is best for targeted analysis with low LOD
• LC-HRMS allows non-targeted screening
• Be aware of the scope of your method and its limitations

Acknowledgements
• The Connecticut Agricultural Experiment Station
• Brian Eitzer
• Elizabeth Lin
• Carlos Tamez
• Jeremy Koelmel
This presentation is not an official endorsement of any products or companies. The opinions
expressed are my own.

Contact Information
Dr. Sara L. Nason
Departments of Environmental Science and Analytical Chemistry
Connecticut Agricultural Experiment Station
Sara.Nason@ct.gov
